We need to do more than ‘better’ – we need differentiated approaches.
 By Jeff Elton, PhD, CEO
By Jeff Elton, PhD, CEO
As we approach the close of the second year of the pandemic, it is clear that we need to redouble our focus on restarting and accelerating the oncology clinical development pipeline. It is one of the hard-won lessons and insights of the COVID-19 period that there are new models of collaboration, data accessibility, AI methods, and digital execution that can bend the legacy cost, time, and productivity curves. While most of these solutions were directed at COVID-19 vaccines and therapeutics, there were tremendous insights generated for oncology trials that we believe are the foundations for a new model.
Cancer patients were disproportionately negatively affected by the COVID-19 virus, especially those with hematological malignancies.i
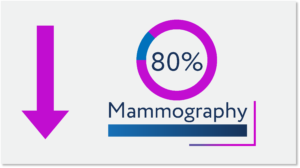
Clinics needed to reduce density of patients and minimize non-essential activities and procedures. Consequently, new patient accruals to clinical studies slowed significantly, new study starts stopped, and patient trial dropouts increased as some patients returned to standard-of-care. Further exacerbating this situation is that new cancer screenings were reduced, delaying when many patients would initiate standard-of-care treatment or find potentially valuable clinical trials.
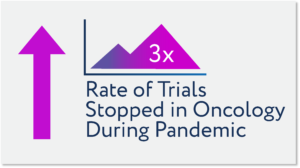
Late last year ConcertAI tracked changes in trial accrual rates and standard of practice modifications, with the conclusion that clinical trial participation rates would be down 60% or more. The number of stopped trials peaked in June 2020, with this level being 3x normal levels and more than half of registration trial levels at the peak.
While the number of stopped trials improved after November 2020, the number enrolling is 30% lower than pre-pandemic with 20 to 30% of stopped trials still suspended as of this summer 2021. Most of the stopped trials were interventional – or those testing new novel entities that might represent treatment for cancers largely unresponsive to standard-of-care treatments. And of the more than 100,000 patients that returned to clinical trials in late 2020 and early 2021, the majority were in studies restarting after slowing or terminating new accruals earlier in 2020.ii The bottom line is that we are still catching-up and forced to prioritize re-initiation of past studies or move to the higher priority studies advancing to the clinic in mid- to late 2021.
How far behind are we?
By trying to ‘catch-up’ using traditional approaches to study design, study locations, patient accruals, and study execution we estimate it will take three or more years to achieve performance equivalent to 2019.
But there is an even bigger imperative in front us. A report issued in July 2021 by the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the North American Association of Central Cancer Registries (NAACCR), and the National Cancer Institute (NCI) noted that between 2014 and 2018, death rates dropped for 11 out of 19 of the most common cancers among men and 14 of the 20 most prevalent cancers among women.iii While some of these declines were a result of improvement in use of existing therapeutics, the major portion is attributable to newly approved therapeutics with novel mechanisms, transforming the standard-of-care and patient outcomes.
So, the rather onerous truth is that study stops, and longer timelines for trial completions, etc. could have the highly negative impact of slowing the improvements in cancer death rates realized over the past decade. We can’t let that happen.
Finding ways forward
The FDA saw the challenges confronting oncology trials emerging and responded swiftly and responsibly.iv This included allowing the flexibility to amend trial processes and use alternative platforms to sustain trial progress while assuring patient safety. The FDA also recognized the growing importance and utility of real-world evidence and digital or decentralized trial approaches.
While these interim approaches were a direct reaction to the challenges facing oncology clinical trials during the pandemic, they were also a statement on coming changes in evidence generation and trial execution technologies and methodologies. This advanced further with the publishing of draft guidance on real-world data for evidence generation in support of regulatory decisions on September 30, 2021. We, like many others in the industry, see this as one of those watershed moments where methodologies and approaches that have been advancing at a modest pace were formally confirmed and now are expected to accompany 100% of oncology regulatory submissions.
Over the next 10 weeks we are going to advance a series of perspectives on the key actions that we can collectively take to assure that the pace of biomedical innovations bringing improvements in cancer deaths and the quality of patient outcomes increase in the coming three to four years.
These short articles will intentionally challenge our industry’s conventional ways-of-working and suggest a new system for evidence generation that brings together real-world evidence-centric approaches with digital and AI-enabled prospective clinical research technologies and methodologies. Our perspective will suggest that new models of biopharma sponsor, healthcare provider, and digital solution providers will be required.
The first of these will build on the September 2021 preliminary guidance from the FDA on generation of real-world evidence in support of regulatory decisions. The second will focus on how we can focus on community care centers as the locus for clinical trial capability building and capacity expansion. The third will discuss how real-world data at scale, combined with AI and machine learning tools, can change trial design and advance studies that are at once more representative of patients receiving treatment, integrative of racial and ethnic representation, and more efficient to execute. The fourth will discuss new AI and digital tools for community trial execution capability-building and focus on using existing clinical infrastructure as the source for all trial data – the principal of “enter once, use many times.” The fifth will integrate the themes of articles one through four into a unified view of the clinical development organization of 2022 – 2025 and make the case for how this new model can increase overall capacity by 30 to 40%. This is critical to the goal of achieving study accelerations and accelerating the accumulation of evidence necessary for new approvals that can allow us to sustain and ultimately improve on the recent reductions in cancer deaths.
Complementing the publication of these articles will be interactive webinar panels early in the new year bringing together healthcare providers, trial sponsors, with digital solution and real-world evidence companies.
We see this as a responsibility and imperative for cancer patients – progress is hard enough to make, losing gains is simply heartbreaking. Together we believe we can move to a new model for biomedical innovations and clinical development with substantially improved cost, execution times, and precision. Not only can we ensure the ongoing reductions in cancer deaths, but we can accelerate this well ahead of the levels achieved in the past 10 years.
i JAMA Oncol. 2021;7(3):458-460. doi:10.1001/jamaoncol.2020.7600; https://jamanetwork.com/journals/jamaoncology/fullarticle/2774867
ii Nature Reviews Drug Discovery 20, 415 (2021); https://www.nature.com/articles/d41573-021-00086-8
iii https://seer.cancer.gov/report_to_nation/?cid=pr_cgov_en_sharedlink_arn_lp
iv JAMA Oncol. 2021;7(1):27-28. doi:10.1001/jamaoncol.2020.4975; https://jamanetwork.com/journals/jamaoncology/fullarticle/2771201
